
Other consortium members
University of Bonn
Germany
Christian Steinhäuser
University of Amsterdam
The Netherlands
Eleonora Aronica
CNR Institute of Neuroscience
Padua, Italy
Giorgio Carmignoto
Cardiff University
United Kingdom
Vincenzo Crunelli
MPI of Experimental Medicine
Göttingen, Germany
Frank Kirchhoff
Eurice GmbH
Saarbrücken, Germany
Claudia Giehl
Brief description and aims of work
Since 1996 our scientific interest has been centered in the study of the processes of communication between astrocytes and neurons, and especifically in the role of astrocytes in controlling neuronal activity. Our work has given rise to novel experimental results, which have helped to demonstrate the existence of bidirectional communication between astrocytes and neurons, the active participation of the astrocytes in neuronal physiology and synaptic transmission, and the cellular and molecular mechanisms involved.
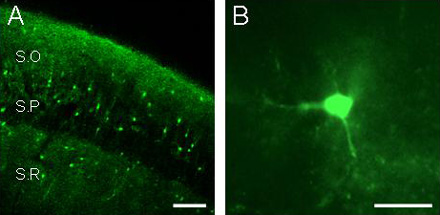
A) Fluorescence image of a fluo-3 filled slice showing the hippocampal stratum radiatum (SR), the stratum pyramidale (SP), and the stratum oriens (SO), obtained by laser-scanning confocal microscopy. Note the lower relative fluorescence at the pyramidal layer. Scale bar, 100 µm. B) Epifluorescence image of a fluo-3 filled hippocampal astrocyte. Scale bar, 20 µm.
Previous findings
In 1998, we demonstrated in cultured preparations that the Ca2+-dependent release of glutamate by astrocytes evoked a miniature synaptic transmission (Araque et al, Eur J Neurosci, 1998. Araque et al, J Neurosci, 1998).
In 2000, we demonstrated that astrocytes release glutamate through Ca2+-dependent and vesicle-associated proteins. (Araque et al, J Neurosci, 2000).
In 2002, we showed that discrete subcellular regions of astrocytes respond to synaptically released acetylcholine. (Araque et al, J Neurosci, 2002).
In 2005, we demonstrated that astrocytes integrate and process synaptic information and confirmed that astrocytes modulate neuronal electrical activity through activation of NMDA receptors (Perea and Araque, J Neurosci, 2005).
In 2007, we showed that astrocytes release adenosine to modulate glutamatergic synaptic transmission during hypoxia.
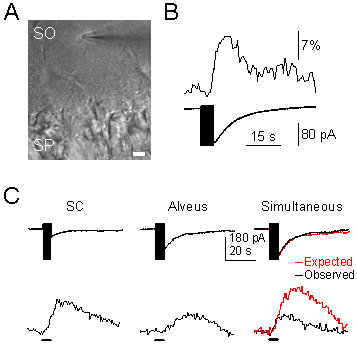
A) Infrared differential interference contrast (DIC) image, showing the hippocampal stratum pyramidale (SP) and the recorded astrocyte in the stratum oriens (SO). Note the recording pipette on the right side of the astrocyte. Scale bar, 20 µm. B) Representative astrocytic Ca2+ levels (upper trace) and whole-cell membrane currents (lower trace) elicited by SC stimulation. The vertical black bar on the current trace corresponds to the stimulus artifact. C) Representative whole-cell currents elicited by independent or simultaneous stimulation of SC and alveus. In simultaneous stimulation, black and red traces correspond to the observed and expected responses (i.e., the summation of responses evoked by independent stimulation of both pathways).
Implications of our research
Recent data have demonstrated the participation of astrocytes on brain physiology, as well as their involvemet in the pathogenesis of some nervous system dysfunctions. Beyond the well-known role of astrocytes in the control of brain homeostasis, the disruption of mechanisms involved in astrocyte-neuron signalling may result in the appearance of some brain diseases. For example, the ability of astrocytes to release neuroactive substances not only results in the active participation of astrocytes in the physiology of the nervous system, but also implies that astrocytes may be responsible for some brain diseases. Therefore, the identification of cellular and molecular mechanisms underlying neuron-astrocyte communication will help to a better understanding of the mechanisms of brain phsyiology as well as to appropriately identify cellular targets to direct therapeutic approaches for the future treatment of several brain diseases.
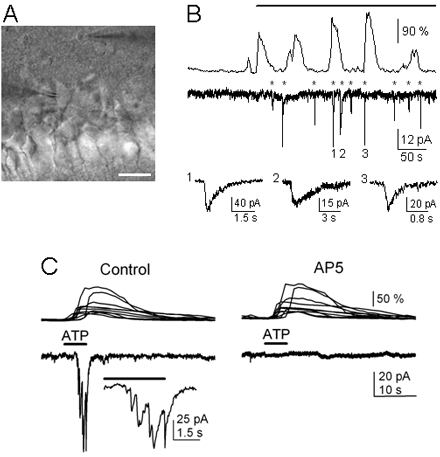
A) Infrared DIC image. Note the recording pipettes on the right side of the astrocyte in the stratum oriens (top) and on the left side of the neuron in the pyramidal layer (bottom). Scale bar, 25 µm. B) Representative astrocyte Ca2+ signal and neuronal current obtained from paired whole-cell recordings in control and after astrocyte stimulation (top horizontal bar) with depolarizing pulses. Asterisks indicate neuronal SICs. Some SICs have been truncated, and three are expanded at the bottom traces. Note the frequency increase of both astrocyte Ca2+ oscillation and neuronal SIC after astrocyte stimulation. C) Astrocyte Ca2+ levels (top traces) and whole-cell neuronal currents (bottom traces) during ionophoretical application of ATP (horizontal bar), in TTX and absence of Mg2+ (control), and after perfusion with 50 mM AP5. Inset, expanded current trace illustrating the multiple NMDAR-mediated SICs.
Past and present collaborations
- Washington Buño · Instituto Cajal, CSIC, Madrid, Spain
- Giorgio Carmignoto · CNR Institute of Neuroscience, Padua, Italy
- Valentín Ceña · Universidad de Castilla La Mancha, Albacete, Spain
- Javier DeFelipe · Instituto Cajal, CSIC, Madrid, Spain
- Rafal García de Sola · Hospital de La Princesa, Madrid, Spain
- Philip G. Haydon · Department of Neuroscience, University of Pennsylvania School of Medicine, Philadelphia, USA
- Mario Vallejo · Instituto de Investigaciones Biomédicas Alberto Sols, CSIC, Madrid, Spain